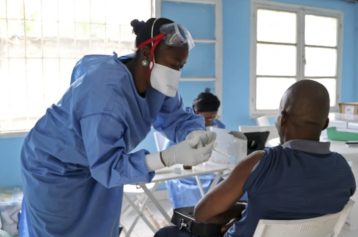
The Ebola “miracle” drug that may have saved the lives of two Americans is now being administered to a Catholic priest from Spain. Meanwhile, West African authorities are still being turned away in their efforts to get the drug for their nations, where the disease has killed more than 700 people.
The revelation about Spanish priest Miguel Pajares, 75, will undoubtedly intensify the controversy over who is getting access to the experimental drug ZMapp and who isn’t. Pajares reportedly was flown from the Liberian capital of Monrovia to Spain last Thursday.
West African leaders have requested the drug from the U.S. Centers for Disease Control and Prevention (CDC), but they say they were told only a few doses were available.
The priest is getting the drug under a Spanish law that allows the “use of non-authorized medications in cases where a patient’s life is in danger and they can’t be treated satisfactorily with an authorized medication,” Spain’s drug safety agency said in a statement that was printed in the Spanish newspaper El Pais.
ZMapp was developed in a partnership between San Diego-based Mapp Biopharmaceuticals, San Diego-based LeafBio, and Defyrus Inc., a biodefense company based in Toronto that collaborates with the U.S. military, according to Forbes.
After the international outcry over the decision to administer the new Ebola “miracle” drug to two white Americans while hundreds of Africans continue to die, the World Health Organization announced that it is meeting this week with medical ethicists to determine how experimental drugs should be administered going forward.
“We are in an unusual situation in this outbreak. We have a disease with a high fatality rate without any proven treatment or vaccine,” Marie-Paule Kieny, assistant director-general at the WHO, said in a statement issued Thursday. ”We need to ask the medical ethicists to give us guidance on what the responsible thing to do is.”
Last week, three of the world’s leading experts on the devastating disease released a joint statement asking why the experimental drug that appears to have miraculously cured two white Americans has not been offered to African patients.
Peter Piot, director of the London School of Hygiene and Tropical Medicine, who discovered Ebola in 1976; David Heymann of the Chatham House Centre on Global Health Security; and Jeremy Farrar of the Wellcome Trust, all said that Africans infected by Ebola should get the same chance as the Americans to be cured.
“African governments should be allowed to make informed decisions about whether or not to use these products – for example, to protect and treat health care workers who run especially high risks of infection,” they wrote in a joint statement.
Ebola has been diagnosed in 1,600 people and has killed more than 900 in Liberia, Sierra Leone and Guinea. It has so far killed about 60 percent of those infected.
A report in The New York Times said the U.S. government also is in the process of forming a group to consider the same issues.
President Barack Obama said Thursday that it was too early to decide whether the experimental drug ZMapp, which appeared to cure American doctor Kent Brantly and missionary Nancy Writebol, could be distributed in Africa.
Obama said that during his summit with African leaders last week they decided to establish an African version of the American Centers for Disease Control and Prevention.
Referring to the WHO, the three Ebola experts said it was “the only body with the necessary international authority” to allow such experimental treatments, and that it “must take on this greater leadership role.”
“These dire circumstances call for a more robust international response,” they said.